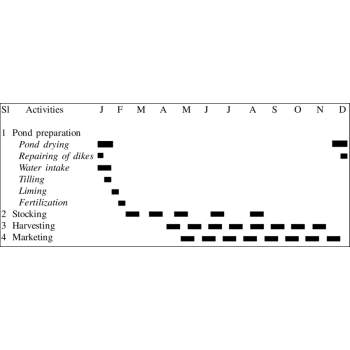
A shrimp crop calendar is a systematic timeline that guides farmers through the stages of shrimp farming, ensuring effective management, healthy shrimp growth, and optimal yields.
It includes activities categorized into pre-stocking, stocking, and post-stocking phases.
1. Pre-Stocking Phase :
Duration: 3–4 weeks
2. Stocking Phase
Duration: Initial stocking to Week 1
3. Post-Stocking Phase
Duration: 3–5 months (depending on shrimp species)
Weekly Management Activities:
4. Mid-Cycle Activities (Month 2–3):
5. Harvesting Phase
Duration: Final Month (Week 18–22)
Advantages of a Shrimp Crop Calendar:

Symptoms
Zoothamnium sp. Infected shrimps show black/ brown gills or appendage discoloration or fuzzy/cottony appearance due to a heavy colony of the organisms. In some cases, the severely affected shrimp die during the molting period.
The base of Zoothamnium forms a circular disc that fuses with the epicuticle but does not penetrate the underlying cuticle or epithelium. Discoloration of the gills is a common symptom of fouling by organisms and also detritus, silt, or iron which are trapped by the fouling organisms.
Treatment
Chlorine and formalin are often used to treat those commensal organisms if shrimp display heavy infection. Changing water is the most preferable management, which stimulates molting of the shrimp in order to reduce the infestation.
Prevention and Control
Prevention and control of the occurrence of surface fouling are usually done through maintenance of good sanitary conditions at the pond bottom and the overall pond area. Organic matters and suspended solids in the pond should be reduced to prevent the attachment of those fouling organisms. This is achieved by changing the water or applying lime.

Infectious Myonecrosis Virus (IMNV) is an emerging shrimp RNA virus causing the disease, infectious myonecrosis (IMN). The disease was first recorded in Pacific white shrimp, P. vannamei in Brazil in 2002 and then in 2006 in Indonesia including Java island. The disease causes significant economic losses to aquaculture due to associated mortalities in P. vannamei in grow out ponds. The virus infects all the life stages of shrimp including post larvae, juveniles, and adult, but the mortality was observed in the juveniles and adult with a cooked appearance.
Causes
IMN is caused by a putative totivirus. IMNV particles are icosahedral in shape and 40 nm in diameter. Transmission via water and vertical transmission from broodstock (trans-ovarian or by contamination of the spawn eggs) to progeny is also likely to occur. IMNV may also be transmitted among farms by feces of seabirds or shrimp carcasses. Outbreaks of IMN with sudden high mortalities may follow stressful events such as capture by cast-net, feeding, sudden changes in salinity or temperature, etc., in early juvenile, juvenile, or adult P. vannamei in regions where IMNV is enzootic.
Symptoms
IMN disease causes significant mortality in grow out ponds and is characterised by acute onset of gross signs including focal to extensive whitish necrotic areas in the striated muscle, especially of the distal abdominal segments and the tail fan, which may become necrotic and reddened similar to the colour of cooked shrimp.
Juveniles and sub-adults of P. vannamei, farmed in marine or low salinity brackish water, appear to be the most severely affected by IMN disease. The principal target tissues for IMNV include the striated muscles, connective tissues, haemocytes and the lymphoid organ parenchymal cells. Severely affected shrimp become moribund and mortalities can be instantaneously high and continue for several days. Mortalities from IMN range from 40 to 70% in cultivated P. vannamei, and FCR of infected populations increase from normal values of ~ 1.5 to 4.0 or higher.
Prevention
No effective vaccines for IMNV are available. IMN can be prevented using SPF broodstock and practicing BMPs.
Why Bacteriophages?
With emergence of highly virulent pathogens in animal health and aquaculture, there is need for highly effective therapeutic product which can reduce usage of sanitisers and antimicrobials. Continuous use of sanitisers and antimicrobials lead to damage of microbiome (the normal non-pathogenic beneficial bacteria) in aquaculture pond ecosystem and shrimp gut. Recent explosion of scientific research has brought to light the importance of maintaining good and unfluctuating microbiome in shrimp and fish health and aquaculture success.
Under this condition Bacteriophages against virulent pathogens in aquaculture will be highly helpful in maintaining the natural ecosystem of the pond while killing the aquaculture pathogens. This strong argument inspired Salem Microbes to initiate Bacteriophage research as early as 2018 and has invested in infrastructure, technology, and technical competence in bacteriophages-based solution development for Aquaculture, Poultry and Food safety.
Salem Microbes possessing 23 years of probiotic tradition has always travelled with Bacteriophages hand in hand from screening to production operations. In the current day scenario, we are ‘embracing the foe as a friend’ to control pathogens of aquaculture. So, we are just jumping sides and exploiting their potential.
Today, we assure that we are in the forefront of bacteriophage research, specialising in isolation, characterisation, and formulation of Phage cocktail within a short turnaround time. Our Research & Development ensures quick screening in case of a sudden disease outbreak to make custom formulation of Bacteriophages from our expansive library of Bacteriophages maintained on-campus.
Phages and Probiotics , a Synergistic combination
Probiotics have played an undeniable role in the environmental management of the shrimp pond and are going to continue to sustain the shrimp ecosystem microbiome, the only catch here is use of quality probiotic inputs which farm owners and technical experts should be aware of.
Probiotics reduce the chances of infection by Competitive Exclusion, but once the critical levels of Vibrio species dominate the environment, it become nearly impossible for probiotic bacteria to exclude Vibrio species. Such critical condition demands quick and sure shot treatment, which is non residual in nature and non-GMO and which does not affect the (microbiome). Here comes the concept of Phage Therapy which is highly specific and quick acting in nature specifically targeting the aquaculture pathogens.
|
SALIENT FEATURES |
Probiotics Application only |
Bacteriophage Application only |
Both Bacteriophages & Probiotics applied condition |
|
Mode of action |
Competitive exclusion of pathogens by exhausting nutrients for the pathogens. |
Works on identified targeted pathogens by killing them by lysis. |
Phages Eradicates Identified pathogens. There by nutrients are available for probiotic microbes. |
|
How it manages pathogens ? |
Dominates Pathogens but when conditions are conducive for pathogens, they cause disease |
Eradicates pathogens thereby reduces frequency of disease. |
Probiotics have a conductive environment to multiply fast & full |
|
|
Long acting |
Self-limiting |
Phages keeps check on fresh entrants |
|
Residual formation |
No Residues |
No Residues |
Phages leaves un-identified Natural Probiotic flora to thrive better |
|
How they really work ? |
Bacteriocin & Enzymatic action |
Cell Lysis of Pathogenic bacteria. |
Phages prevents horizontal transmission of toxin bearing plasmids. |
|
Source |
Non-GMO, Nature isolates |
Non-GMO, Nature isolates |
|
|
Traceability and proprietary nature |
|
Identified & Genotyping done |
|
|
Is Product Upgradation possible ? |
Continuous Improvement in Performance based on the new strains introduced. |
Continuous update of Phages for New Identified Pathogens makes it a safe therapeutic product with broad spectrum of action. |
|
|
Limitations |
Fights with unknown pathogens |
For Greater Benefit – It’s recommended to use Probiotics after this to dominate the environment and maintain positive balance for maximum results. |
|
|
|
Virulent pathogens with Toxins continue to exist, multiply and cause infections. |
Virulent pathogens with toxins are destroyed, thus halting their propagation. |
|
How a Bacteriophage product become relevant to a condition?
A Good Bacteriophage consortia should contains identified lytic phages, specific against pathogens selected from the target environment. Phage cocktails against Pathogens should be continuously upgraded to maintain the pathogen spectrum, specificity and infectivity.
How does a Phage works?
Bacteriophages are Viruses of bacteria which enter the bacteria, use the bacterial source of energy, hijack the bacteria, multiply inside the bacteria and kill them by lysis. When they kill the bacteria, each virus which enter the bacteria multiplies and releases 10 to 100 mature ready-to-attack bacteriophages which are capable of infecting and destroying next set of target pathogenic bacteria.
V PHAGES Consortia
V PHAGES Cocktails for Hatchery and Growout are quite different in their Formulation, Specificity and Purpose. V PHAGES HATCHERY satisfy the need for targeting pathogens specific and prevalent in Hatchery water source, live feeds and also its environment. V PHAGES GROWOUT targets the common pathogens esp Vibrios which are the main cause for bringing down the productivity of a shrimp pond by causing Vibriosis leading to White gut, White faecal disease, off feed, Running Mortality Syndrome and crop loss.
"V PHAGES " cocktails target Vibrio species as,
When TO USE IN SHRIMP HATCHERIES?
When TO USE IN SHRIMP GROWOUT PONDS
Comparing with Other Therapies
V PHAGES are Natural isolates screened and selected for their strong and fast action against their challenged targets. They do not disturb the Probiotics and other non-pathogenic natural microbiome which are otherwise destroyed by other treatment approaches as Sanitisers, Disinfectants and any products of anti-microbial action. These Phages does not leave any residues on the shrimp or environment as they are Natural. All bacteriophages in V PHAGES cocktail are Genotypically characterized to ensure proprietary formulation and traceability, which makes them special.
Conclusion
Though Bacteriophage research and applications are known for many years very few products have seen the light of the day. Even these products become irrelevant when they are not supported by a strong expertise in the field and research infrastructure. Salem Microbes has established these capabilities and commits to be in the forefront of giving the Best to its farmers to make their Shrimps Healthy, Productive and Profitable.

Black gills, black gill syndrome, black gill disease, or melanization of the gills is symptomatic of several causes of gills disease. Shrimp Black Gill Disease has been one of the most frequent shrimp farming problems and caused many deaths, especially in the late rearing period. There are infectious and noninfectious causes of black gill disease. Infectious causes include Fusarium solani, Vibrios, and Lagenophrys, while noninfectious causes include nutrient deficiencies and exposure to pollutants and contaminants.
A number of abiotic and biotic reasons have been attributed to the black gill in shrimps. Presence of excessive levels of toxic substances such as nitrite, ammonia, heavy metals, crude oils etc. in the culture water may lead to black gill disease. High organic load, heavy siltation and reducing conditions in rearing pond can also cause this disease in shrimps. Attack of certain bacterial, fungal and protozoan pathogens can also cause black gill condition in shrimp.
Causes:
Black gills in shrimp can be caused by several things.
Symptoms:
Prevention:
Remedies:
Treatment of the black gill disease depends upon the cause of the disease.

Secchi Disc Measurement
Application of zeolites and water exchange are routine practices in shrimp culture to control the turbidity.

Running mortality syndrome (RMS) is named by shrimp farmers for continuous low-level mortalities during the culture period, resulting in low survival and productions. The syndrome is widely prevalent in the vannamei farms since 2011 in Andhra Pradesh and Tamil Nadu. The affected shrimp show patches of whitish musculature in the junctions of 2nd and 4th abdominal segments as a clinical sign with continuous low-level mortalities. This condition results a small percent shrimp mortality in the affected pond on daily basis. As the mortality continues on daily basis till the rest of the culture period, it is called as “Running Mortality Syndrome (RMS)”. Usually the RMS started after 35–40 days of culture (DOC) with low mortalities and as the culture progressed, the mortality rate also increased and the problem becoming acute at around 90 DOC and the farmers were forced to prematurely harvest the crops.
Shrimp from RMS affected ponds tested negative for all the major OIE listed and other pathogens such as White Spot Syndrome Virus (WSSV), Infectious Hypodermal and Hematopoietic Necrosis Virus (IHHNV), Monodon Baculovirus (MBV), Hepatopancreatic Parvo Virus (HPV), Infectious Myonecrosis (IMNV), Taura Syndrome Virus (TSV), Yellow Head Virus (YHV), and Penaeus vannamei Noda Virus (PvNV).
Causes:
Bacteriological examination of haemolymph and hepatopancreas samples of RMS affected shrimp indicated predominance of Vibrio spp., such as Vibrio parahaemolyticus and Vibrio azureus.
Symptoms:
In early stages of this condition, Litopenaeus vannamei, show certain gross symptoms. These include:
Management:
Running Mortality Syndrome is depicted be pond management associated syndrome rather than infectious in nature and thus can be overcome through best management practices.
In the beginning days, farmers managed this disease by regularly removing the dead shrimp from the pond. Reducing the stocking density by partial harvesting reduced the mortality. Reduced feed quantity or suspending feed for few days reduce the mortality.
The culture practices like stocking bigger post larvae, Avoid over stocking, nursery management, Regularly monitor water quality, health of animals, strict feed management and partial harvest will reduce the incidence of RMS.
Treatment:
During the onset of Running Mortality, very few dead pieces were found in the pond, a very much advised of V Phages Growout to control Vibrio sp. in the pond. It helps to drastically reduce the Running Mortality Syndrome. It is always advised to apply Bacteriophages as a preventive strategy to avoid RMS conditions caused by Vibriosis.

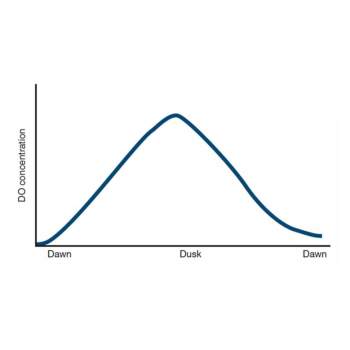
Low level of oxygen concentration can be caused by a number of reasons
Some symptoms of shrimp when water with dissolved oxygen is lacked:
Shrimp concentrates near water surface, edges of ponds, near position where water comes in; lethargic shrimp with strong respiratory rate, coma and possible death in shrimp.
Effects of DO in shrimps:
|
Dissolved oxygen (ppm) |
Effects on shrimp |
|
0.3 |
Shrimp die |
|
1.0 |
Anoxia in shrimp, shrimp may die |
|
2.0 |
Shrimp cannot grow up |
|
3.0 |
Shrimp grows slowly |
|
4.0 |
Shrimp grows normally |
|
5.0 – 7.0 |
Shrimp grows healthily and rapidly |
How to overcome the lack of DO in the pond?

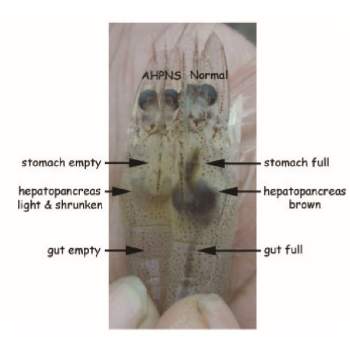
Acute hepatopancreatic necrosis disease (AHPND) earlier known as early mortality syndrome (EMS) or acute hepatopancreatic necrosis syndrome (AHPNS) has been causing significant losses in shrimp farms in China, Vietnam, Malaysia, and Thailand since 2009. The disease affects both black tiger shrimp and Pacific white shrimp and is characterized by mass mortalities during the first 20-30 days of stocking.
Research by the University of Arizona has identified that the disease is caused by the bacterial agent Vibrio parahaemolyticus, which is transmitted orally and colonizes the shrimp’s gastrointestinal tract. This then produces a toxin that causes tissue destruction and dysfunction of the shrimp digestive organ known as the hepatopancreas.
Causes
The disease is caused by a unique strain of Vibrio parahaemolyticus, which can produce toxins responsible for the primary pathology in affected shrimp. Other non-Parahaemolyticus strains such as V. campbellii, V. harveyi, V. owensii, and V. punensis are also found to contain the same toxic genes and may also cause the disease.
Symptoms
Management
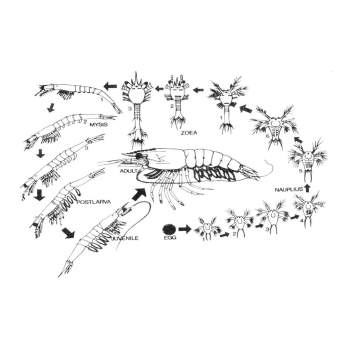
Identification of different larval stages is of much importance in the successful operation of a hatchery. As the different larval stages need different types of food and water quality environment, it becomes necessary to identify and segregate them from other groups. Penaeid Shrimps pass through three larval stages before attaining the postlarval stage i.e., nauplius, zoea, and mysis.
Follow the points given below to identify the larval stages of shrimps.
Nauplius stages:
Protozoea stages:
The body of the zoea is more elongated than the nauplius. The body consists of the carapace, thorax and abdomen. They have functional digestive tract and filter feeders. They feed mainly on unicellular algae present in the medium.
It has 3 sub stages, Protozoea I, II and III. The early protozoea stage has a pair of protruded compound eyes, the next stage is characterized by the presence of a rostrum and the late protozoea stage has a pair of uropods.
The stages lasts for 3-4 days and is succeeded by Mysis stage.
Mysis stages :
Mysis swims through flexing their abdomen with the head pointing downwards.
It also has 3 sub stages:
M I - No pleopod bud
M II - Pleopod bud appears
M III - Pleopod bud with 2 segments
This stage lasts for 3-4 days and then pass to I post larval stage.
Post Larva:
The postlarva resembles the adult shrimp and it can be distinguished from the mysis stage by the presence of setae on the pleopods which are functional. The postlarvae also prefer to cling to the solid surfaces and the walls of the tank. Substages of postlarvae are called as PL 1, PL 2, PL 3 etc., wherein the number indicates the number of days as postlarva. Postlarvae feed on zooplankton and other dead organic matter. They can tolerate lower salinities.

White spot disease (WSD) is the most serious threat faced by the shrimp farming industry worldwide. WSD was first reported in farmed P. japonicus from Japan in 1992-93, but was thought to have been imported with live infected post-larvae from mainland China. The disease is transmitted vertically from infected brood stock to larvae and horizontally either by ingestion of infected organisms or through carrier organisms. Most crustaceans including all penaeid shrimps (monodon, vannamei, indicus etc.) and crabs can be affected by WSD. All the life stages of shrimp may get infected by this virus.
Causes
White spot disease is caused by a virus called White Spot Syndrome Virus (WSSV). It is a rod-shaped double-stranded DNA virus of 120-150 x 270-290 nm size, assigned to a new virus family, whispoviridae.
Symptoms
Diagnosis
WSD may be diagnosed based on gross signs such as the presence of the characteristic white spots, and rapid mortalities. White spots may not be always seen in the early stages of infection in shrimp. WSSV can be detected using polymerase chain reaction (PCR), or with molecular tools such as dot-blot and in situ hybridisation (ISH) tests.
Prevention

Every living thing has to feed to survive. They either act as predators or become prey. Shrimps are preyed upon by birds. They preyed on shrimps and reduce the population of the livestock in the pond or water body. Shrimp farmers go through such losses on a yearly basis. They end up losing a large amount of their investment to predator attacks and because of poor management practices. Bird fencing should be checked daily, if any errors that should be corrected in time to protect the crop. It is necessary, because birds negatively affect shrimp production by transmitting or transporting diseases, weed seeds and parasites from pond to pond or from one facility to another.
Using plastic netting for growing shrimps is effective. Netting makes a great predator barrier.

Shrimp of the family Penaeidae follow a similar body design to that of most malacostracans. That is, they are laterally compressed, elogate decapods, with a well-developed abdomen adopted for swimming. Each segment is enclosed by a dorsal tergum and ventral sternum.
Shrimp’s body can be separated into two parts. The upper portion of the shrimp is called as cephalothorax and the lower portion is the abdomen segment. The main characteristics of decapods is having an exoskeleton, the shell which is periodically shed (molting) to allow further growth.
Cephalothorax
Abdomen